About Me
Who is Noelle James?
As a combined Post Doc and Lab Manager in the Auerbach Lab, I am using rats to learn how brains think about sound. We use response speed, a behavior, to ask how loud you or a rat think a sound is. In our rats, we use the same tests to see how hearing works or doesn’t when you have perception issues, like hearing loss or Autism.
I graduated from the Neuroscience program at the University of Illinois in December 2019. I did my graduate research in Dr. Alison Bell’s animal biology lab. Prior to grad school, I worked as a technician/lab manager for 6 years in Dr. Phil Newmark’s molecular and cellular biology lab. My undergrad major was Psychology at Washington University in St. Louis. If you want to know more, you can check out my CV.
Read about specific projects or a brief summery of my thesis. Complete datasets for some projects can be found on the open science framework.
My main research interests are behavioral genetics and social neurogenesis.
Research
I have worked with:
| Rat | Threespine stickleback fish | Planarians | Fruit flies | |||
|---|---|---|---|---|---|---|
 |
 |
 |
 |
|||
Not pictured: Toxoplasma gondii, E. coli, freshwater snails
Using behavior to look at how the brain helps us hear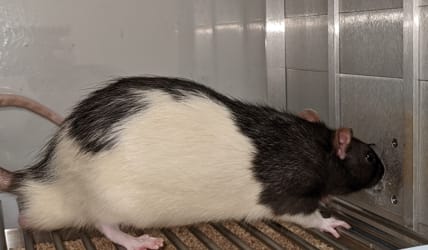
We can respond faster to things that are easier to hear (sense). This lets us use speed, a behavior, to ask how loud you think a sound is. In our rats, we use the speed and accuracy to see how hearing works or doesn’t when you have perception issues, like hearing loss or Autism. By combining the behavioral test with other molecular and electrophysiology tools, we can find specific locations and even cells in the brain that play a role in hearing.
By learning how brains think about sound, we can find better treatments for problems like when sound is so loud its painful or distracting. We are also trying to figure out at what age and where in the brain treatment can be the most effective.
Altering behavioral gene expression in the brain: Viral-mediated transgensis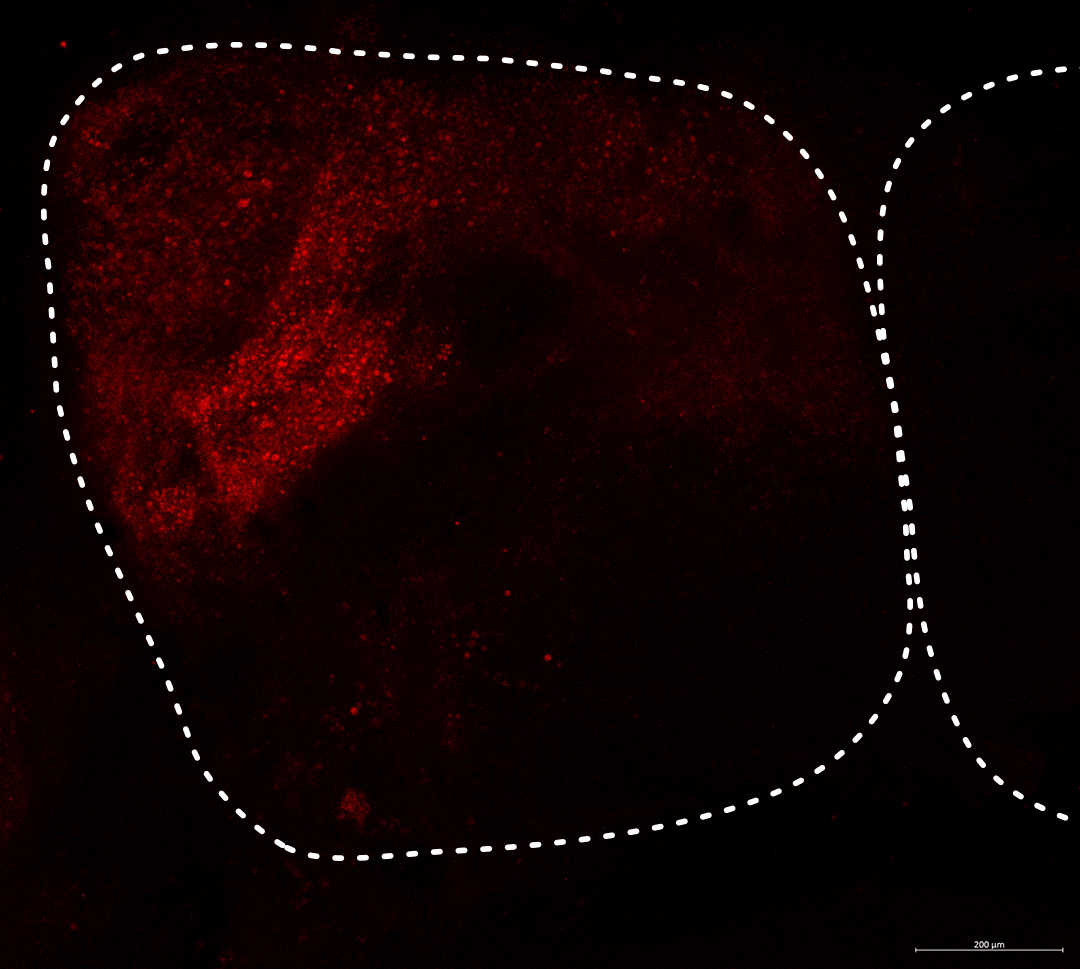
Research Article (PLoS One 2021)
One method to test the role a gene has in the brain is to over express it. By looking for a change in behavior after altering the gene’s expression we can see what role it plays. For example, if we increase the expression of gene MAOA and then the fish bites or charges more at other fish, it suggests that MAOA is important for aggression.
I am particularly interested in doing this in adult animals as many genes that are used in during childhood development are used for completely different things later in life. Additionally, in animals, we can use naturally-reared, wild-caught animals which reduces the effects of artificial rearing. We also want to have this effect be transient so that we can limit the effect to a short but important time period - like during parenting but not during conception or visa versa.
Talks:
- Behaviour 2019
- Emerging Researchers National (ERN) Conference 2018 - 1st Place Graduate Oral Presentation in Biological Sciences
Posters:
- Carl Woese Institute for Genomic Biology Fellows Symposium - Best poster award
- Society for Neuroscience (SfN) 2019 Annual Conference - Dynamic Poster (a cross between a talk and poster)
- Society for Neuroscience (SfN) 2018 Annual Conference
Social Neurogenesis
 There is a lot of variation in the ability of animals to make new brain cells, called neurogenesis, as adults. Some animals, like planarians, can make a whole new brain from scratch. Others, like humans, have very limited ability to make new brain cells that is restricted to specific regions of the brain. Sticklebacks are capable of making more brain cells than humans and increase their brain size throughout their life.
There is a lot of variation in the ability of animals to make new brain cells, called neurogenesis, as adults. Some animals, like planarians, can make a whole new brain from scratch. Others, like humans, have very limited ability to make new brain cells that is restricted to specific regions of the brain. Sticklebacks are capable of making more brain cells than humans and increase their brain size throughout their life.
Many situations can change the rate that new brain cells are made. Things like stress and social isolation reduce the rate while things like exercise and enriched environments can enhance the rate. I am interested in how an event like successfully defending a territory can influence the rate of neurogenesis.
FISH on Fish - In situ Hybridization
Protocol Paper (Evolutionary Ecology Research 2016)
In situ hybridization is a technique that allow you to visualize where a gene is being expressed. The location can be determined at the level of the cell. We use this technique to link behavioral variation (such as aggression levels) with changes in where genes are being expressed. Ideally this allows us to identify regions of the brain that participate in the behavioral response.
3 Minute Thesis talk
 While we scientists no longer argue over nature versus nurture, we still don’t really understand how both come together to give rise to the amazing variety of behavior we see. Social interaction underlies many of these behavior including aggression. While typically thought of in a negative light, aggression is not only required, but sometimes desirable - such as in boxing, the military, and protecting our families. In fact, it’s only when aggression appears in wrong situations or levels that its bad.
While we scientists no longer argue over nature versus nurture, we still don’t really understand how both come together to give rise to the amazing variety of behavior we see. Social interaction underlies many of these behavior including aggression. While typically thought of in a negative light, aggression is not only required, but sometimes desirable - such as in boxing, the military, and protecting our families. In fact, it’s only when aggression appears in wrong situations or levels that its bad.
I study how genes can tune levels of aggression. The main way of connecting genes to behavior is to ‘catch the gene in the act’ by looking at how expression changes after the fact. However, this really doesn’t get at how the gene is helping shape the behavior in the first place. To show a direct and causal role for genes in aggression, I developed a method called viral-mediated transgenesis for stickleback fish. I use a modified herpes virus to add extra copies of my genes to the brain.
Think of it like a self-driving car. The destination is the cells we want to influence and our vehicle is something that can easily get into those cells. However, I replace the normally harmful herpes passenger with our genes related to aggression and allow it to go to its pre-programed destination. By doing this, I can compare how the same fish fights off the same intruder before and after increasing gene expression. This let’s me directly examine how the gene is contributing to behavior. Basically I turn the gene’s volume up to 11 and look at how grumpy the fish is.
The most interesting gene we have looked at so far is monoamine oxidase, which is well known for being related to depression. Because low levels cause increased aggression, we expected that overexpressing it would decrease aggression. However we found the opposite - increasing gene expression also increased aggression. This suggests that monoamine oxidase has an ideal or goldilocks range - with both too much or too little resulting in the same inappropriate levels of aggression. So now thanks to modified viruses we know more about how genes contribute to behavior.